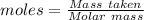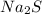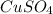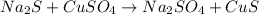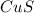What is the percent yield of cus for the following reaction given that you start with 15.5 g of na2s and 12.1 g cuso4? the actually amount of cus produced was 3.05 g. reaction: na2s + cuso4 → na2so4 + cus (a) 16.1% (b) 42.1% (c) 18.93% (d) 7.25% (e) not enough information

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 23.06.2019 00:30, Keemdadream13
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 24.06.2019 00:00, MathChic68
What did ernest rutherford’s gold foil experiment demonstrate about atoms?
Answers: 1
Do you know the correct answer?
What is the percent yield of cus for the following reaction given that you start with 15.5 g of na2s...
Questions in other subjects:

Biology, 25.06.2019 11:00



Physics, 25.06.2019 11:00

Mathematics, 25.06.2019 11:00

Mathematics, 25.06.2019 11:00

English, 25.06.2019 11:00


Health, 25.06.2019 11:00