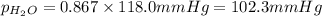Chemistry, 26.09.2019 19:00, Tyrant4life
What is the equilibrium partial pressure of water vapor above a mixture of 62.9 g h2o and 33.2 g hoch2ch2oh at 55 °c. the partial pressure of pure water at 55.0 °c is 118.0 mm hg. assume ideal behavior for the solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, KarenH3512
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 23.06.2019 04:40, yayamcneal05
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
Do you know the correct answer?
What is the equilibrium partial pressure of water vapor above a mixture of 62.9 g h2o and 33.2 g hoc...
Questions in other subjects:

History, 20.09.2019 15:30

English, 20.09.2019 15:30


English, 20.09.2019 15:30

Chemistry, 20.09.2019 15:30

Mathematics, 20.09.2019 15:30

Mathematics, 20.09.2019 15:30



Biology, 20.09.2019 15:30


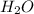 is 102.3 mmHg.
is 102.3 mmHg.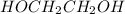 = 33.2 g
= 33.2 g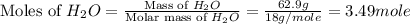
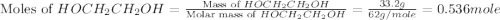
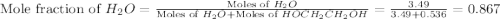
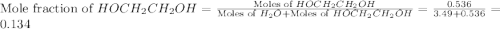
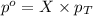
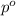 = partial pressure of water vapor
= partial pressure of water vapor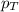 = total pressure of gas
= total pressure of gas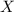 = mole fraction of water vapor
= mole fraction of water vapor