
Chemistry, 26.09.2019 18:00, zoeatlowapple
Consider the reaction between sodium metal and fluorine gas to form sodium fluoride. using oxidation states, how many electrons would each sodium atom lose, and how many electrons would each fluorine atom gain? #e- each sodium atom would loose? #e- each fluorine atom would gain? how many sodium atoms are needed to react with one fluorine molecule?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, bbombard21
Select the atomic models that belong to the same element
Answers: 2

Chemistry, 23.06.2019 02:00, Robloxdemonduckyt
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2

Chemistry, 23.06.2019 02:00, FailingstudentXD
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 06:10, tammydbrooks43
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1
Do you know the correct answer?
Consider the reaction between sodium metal and fluorine gas to form sodium fluoride. using oxidation...
Questions in other subjects:




Mathematics, 22.04.2020 22:15



Mathematics, 22.04.2020 22:15

Mathematics, 22.04.2020 22:15

Biology, 22.04.2020 22:15


![[Ne]3s^1](/tpl/images/0265/3273/4a392.png) .
.
![[He]2s^22p^5](/tpl/images/0265/3273/da8fe.png) .
.
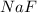 .
.




