
Chemistry, 26.09.2019 16:30, costel8532
An insulated thermos contains 150 g of water at 87.3 ˚c. you put in a 10.2 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 01:30, jonmorton159
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1


Chemistry, 23.06.2019 13:00, nerikzagallegos
Johnny's bakery has 30,900 grams of sugar. a recipe calls for 32 pounds of sugar to be used. how much sugar will be left over? (1 lb=453.59 g).
Answers: 2

Chemistry, 23.06.2019 13:10, zuleromanos
When can a hypothesis be elevated to the status of a theory? a) when it is validated by an experiment b) when data gathered from an experiment precisely fits predictions c) when it can be proved to be true d) when it meets the test of repeated experimentation
Answers: 2
Do you know the correct answer?
An insulated thermos contains 150 g of water at 87.3 ˚c. you put in a 10.2 g ice cube at 0.00 ˚c to...
Questions in other subjects:




Mathematics, 22.10.2020 18:01

Biology, 22.10.2020 18:01


Mathematics, 22.10.2020 18:01


Advanced Placement (AP), 22.10.2020 18:01

Biology, 22.10.2020 18:01


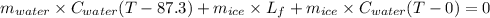
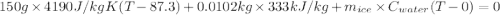
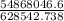
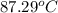
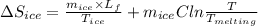
![\frac{0.0102 [\frac{333000}{273.15} + 4190 \times ln (\frac{360.44}{273.15})]](/tpl/images/0265/1324/75975.png)
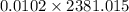
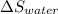 ) =
) = 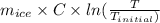
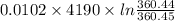
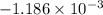 J/K
J/K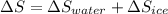
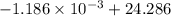 J/K
J/K



