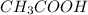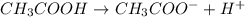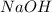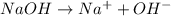Which statement best describes the conductivity of weak electrolytes, such as weak acids and bases?
a) weak electrolytes do not dissolve in water and can not conduct electricity.
b) weak electrolytes dissolve and partially dissociate in water providing charged ions to conduct electricity.
c) weak electrolytes dissolve and completely dissociate in water providing charged ions to conduct electricity.
d) weak electrolytes dissolve and does not dissociate in water providing no charged ions to conduct electricity.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, giraffegurl
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2
Do you know the correct answer?
Which statement best describes the conductivity of weak electrolytes, such as weak acids and bases?...
Questions in other subjects: