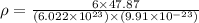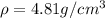Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 22:30, SavageKidKobe
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
Do you know the correct answer?
Titanium has an hcp unit cell for which the ratio of the lattice parameters cais 1.58. if the radius...
Questions in other subjects:

Social Studies, 28.07.2019 08:00


English, 28.07.2019 08:00

Social Studies, 28.07.2019 08:00


Geography, 28.07.2019 08:00

Health, 28.07.2019 08:00


Mathematics, 28.07.2019 08:00

Mathematics, 28.07.2019 08:00


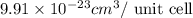
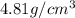
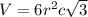
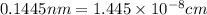
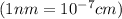
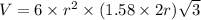
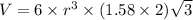
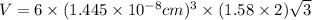
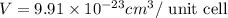
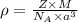
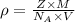 ..........(1)
..........(1)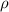 = density of Ti = ?
= density of Ti = ?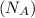 = Avogadro's number =
= Avogadro's number = 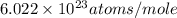
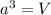 = volume of unit cell =
= volume of unit cell =