
Chemistry, 25.09.2019 02:20, lexizamora2
1.00 kg of ice at -10 °c is heated using a bunsen burner flame until all the ice melts and the temperature reaches 95 °c. a) how much energy in kj is required to effect this transformation?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2
Do you know the correct answer?
1.00 kg of ice at -10 °c is heated using a bunsen burner flame until all the ice melts and the tempe...
Questions in other subjects:

World Languages, 18.10.2020 16:01


Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01


Geography, 18.10.2020 16:01


History, 18.10.2020 16:01

Health, 18.10.2020 16:01


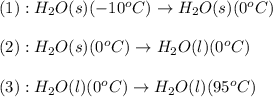
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]](/tpl/images/0260/0438/5cd06.png)
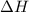 = energy required = ?
= energy required = ?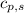 = specific heat of solid water =
= specific heat of solid water = 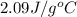
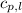 = specific heat of liquid water =
= specific heat of liquid water = 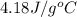
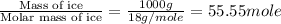
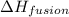 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole![\Delta H=[1000g\times 4.18J/gK\times (0-(-10))^oC]+55.55mole\times 6010J/mole+[1000g\times 2.09J/gK\times (95-0)^oC]](/tpl/images/0260/0438/a7596.png)
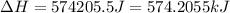 (1 KJ = 1000 J)
(1 KJ = 1000 J)



