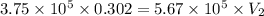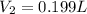Chemistry, 25.09.2019 01:20, JimmySample7
Acompressed cylinder of gas contains 45.6 mol of n2 gas at a pressure of 3.75 x 105 pa and a temperature of 23.6°c. what volume of gas has been released into the atmosphere if the final pressure in the cylinder is 5.67 x 105 pa? assume ideal behavior and that the gas temperature is unchanged.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 22.06.2019 23:30, bxymichelle
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 05:10, citlalli30
Name a brittle metal , which is used to galvanize iron
Answers: 1
Do you know the correct answer?
Acompressed cylinder of gas contains 45.6 mol of n2 gas at a pressure of 3.75 x 105 pa and a tempera...
Questions in other subjects:




Mathematics, 21.10.2020 01:01

Social Studies, 21.10.2020 01:01



Mathematics, 21.10.2020 01:01

Mathematics, 21.10.2020 01:01

Social Studies, 21.10.2020 01:01


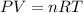
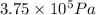 = 3675 atm (1 kPa= 0.0098 atm)
= 3675 atm (1 kPa= 0.0098 atm)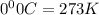
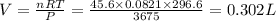
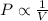 (At constant temperature and number of moles)
(At constant temperature and number of moles)
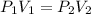
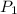 = initial pressure of gas =
= initial pressure of gas = 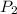 = final pressure of gas =
= final pressure of gas = 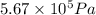
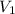 = initial volume of gas = 0.302 L
= initial volume of gas = 0.302 L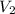 = final volume of gas = ?
= final volume of gas = ?