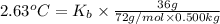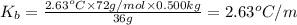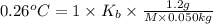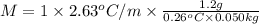The benzene boiling temperature (c6h6) is 80.1ºc dissolving 36 g pentane, c5h12 at 500 g benzene increases the boiling point of the solution to 82.73ºc
a. consider the benzene boiling point constant. show calculations.
b. in dissolving 1.2 g of unknown solute in 50 g of benzene, a solution with a boiling point of 80.36ºc is obtained, which is the molar mass of the solute (assume that i = 1) (show calculations)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1


Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Do you know the correct answer?
The benzene boiling temperature (c6h6) is 80.1ºc dissolving 36 g pentane, c5h12 at 500 g benzene inc...
Questions in other subjects:











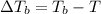
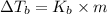
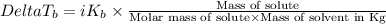
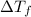 =Elevation in boiling point
=Elevation in boiling point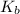 = boiling point constant od solvent= 3.63 °C/m
= boiling point constant od solvent= 3.63 °C/m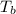 =82.73°C
=82.73°C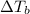 = 82.73°C - 80.1°C = 2.63°C
= 82.73°C - 80.1°C = 2.63°C