
Agas mixture of 20 moles nitrogen and 70 moles hydrogen is fed to a reactor in which ammonia is produced. assuming that the reaction proceeds to completion, determine the composition of the exit gas stream both in mole and mass %. if 75% of nitrogen gets converted, what will be the corresponding fractions?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3

Do you know the correct answer?
Agas mixture of 20 moles nitrogen and 70 moles hydrogen is fed to a reactor in which ammonia is prod...
Questions in other subjects:

Mathematics, 13.02.2021 04:10


Mathematics, 13.02.2021 04:10

Mathematics, 13.02.2021 04:10

English, 13.02.2021 04:10

Mathematics, 13.02.2021 04:10

Mathematics, 13.02.2021 04:10


English, 13.02.2021 04:10

Mathematics, 13.02.2021 04:10


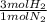 = 60 moles of H₂(g)
= 60 moles of H₂(g) 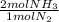 = 40 moles of NH₃(g)
= 40 moles of NH₃(g)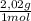 = 20,2 g of H₂
= 20,2 g of H₂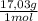 = 681,2 g of NH₃
= 681,2 g of NH₃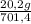 ₓ 100 = 2,9% H₂;
ₓ 100 = 2,9% H₂; 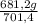 ₓ100 = 97,1% NH₃
ₓ100 = 97,1% NH₃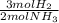 = 45 moles of H₂(g)
= 45 moles of H₂(g) 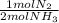 = 15 moles of N₂(g)
= 15 moles of N₂(g) 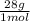 = 140 g of N₂
= 140 g of N₂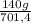 ₓ 100 =20% N₂;
ₓ 100 =20% N₂; 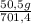 ₓ 100 = 7,2% H₂;
ₓ 100 = 7,2% H₂; 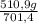 ₓ100 = 72,8% NH₃
ₓ100 = 72,8% NH₃



