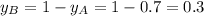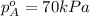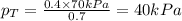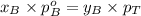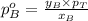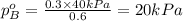Components a and b form ideal solution. at 350 k, a liquid mixture containing 40% (mole) a is in equilibrium with a vapour containing 70% (mole) a. if the vapour pressure of a at 350 k is 70 kpa, what is the vapour pressure of b? (b) 20 kpa (d) 12 kpa (а) 25 kpa (c) 40 kpa

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2



Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3
Do you know the correct answer?
Components a and b form ideal solution. at 350 k, a liquid mixture containing 40% (mole) a is in equ...
Questions in other subjects:











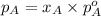 .............(1)
.............(1)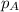 = partial vapor pressure of A
= partial vapor pressure of A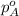 = vapor pressure of pure substance A
= vapor pressure of pure substance A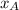 = mole fraction of A
= mole fraction of A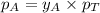 .............(2)
.............(2)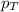 = total pressure of the mixture
= total pressure of the mixture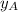 = mole fraction of A
= mole fraction of A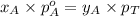
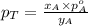 ............(3)
............(3)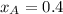 and
and 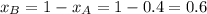
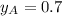 and
and