
Chemistry, 25.09.2019 01:00, nene3210204
Calculate the pressure exerted by ar for a molar volume 0.45 l at 200 k using the van der waals equation of state. the van der waals parameters a and b for ar are 1.355 bar dm mol-2 and 0.0320 dm3mol? , respectively. write your answer (unit: bar) with 2 decimals, as 12.23. do not add unit to your answer.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:50, kelli151
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2


Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1
Do you know the correct answer?
Calculate the pressure exerted by ar for a molar volume 0.45 l at 200 k using the van der waals equa...
Questions in other subjects:





Mathematics, 16.10.2020 06:01


History, 16.10.2020 06:01


Mathematics, 16.10.2020 06:01


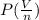 = RT
= RT 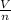 = v; which is called molar volume
= v; which is called molar volume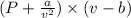 = RT
= RT
 = 0.08314
= 0.08314 






