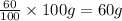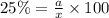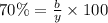Chemistry, 25.09.2019 00:10, 19wawrzkeek
Considering an alloy of the two soluble components a and b. determine the masses of the alloy that are in the liquid and solid phases at a given temperature, if the total mass of alloy is 100 g, component b represents 60 % of the alloy, 25 % of the liquid is component b, and 70% of solid is component b.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1


Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2
Do you know the correct answer?
Considering an alloy of the two soluble components a and b. determine the masses of the alloy that a...
Questions in other subjects:


Mathematics, 10.03.2021 20:40

Arts, 10.03.2021 20:40

Chemistry, 10.03.2021 20:40

Social Studies, 10.03.2021 20:40