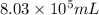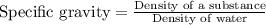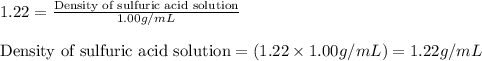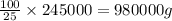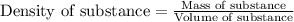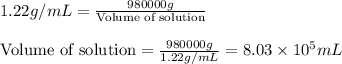Chemistry, 24.09.2019 23:20, SoccerHalo
An aqueous solution of sulfuric acid has a composition of 25.0 wt% sulfuric acid and a specific gravity of 1.22. calculate the volume of solution that contains 245 kg of sulfuric acid.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 21:30, sullivanjakob
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
Do you know the correct answer?
An aqueous solution of sulfuric acid has a composition of 25.0 wt% sulfuric acid and a specific grav...
Questions in other subjects:

Mathematics, 24.08.2020 04:01


Mathematics, 24.08.2020 04:01

History, 24.08.2020 05:01

Health, 24.08.2020 05:01

English, 24.08.2020 05:01


English, 24.08.2020 05:01


Mathematics, 24.08.2020 05:01