Chemistry, 24.09.2019 23:20, maskythegamer
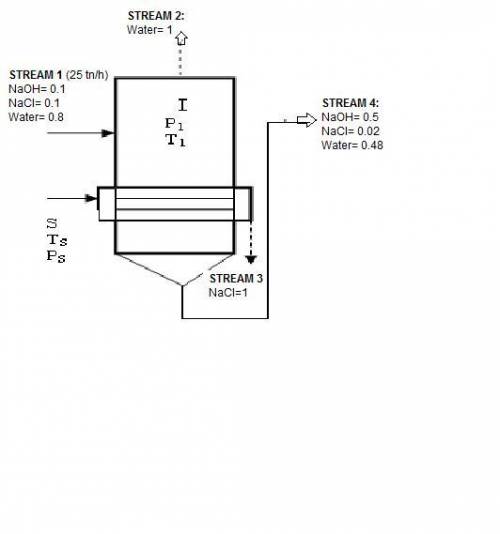
In an evaporator 25 ton / h of a solution of 10% naoh, 10% nacl, and 80% water by weight. during evaporation, the water evaporates and the salt precipitates like crystals they are allowed to settle and are removed. the outgoing concentrated solution of the evaporator contains 50% naoh, 2% nacl and 48% water. based on this information is requested:
1. draw the process flow diagram, indicating each of its streams and compositions (known and unknown).
2. calculate the kilograms of precipitated salt and the kilograms of solution concentrated for every hour of work.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
Do you know the correct answer?
In an evaporator 25 ton / h of a solution of 10% naoh, 10% nacl, and 80% water by weight. during eva...
Questions in other subjects:

Chemistry, 04.11.2021 08:50

Mathematics, 04.11.2021 08:50

History, 04.11.2021 08:50


Mathematics, 04.11.2021 09:00

Mathematics, 04.11.2021 09:00

Mathematics, 04.11.2021 09:00



Mathematics, 04.11.2021 09:00