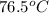Chemistry, 24.09.2019 20:00, andrecoral105
Amixture of 454 kg of applesauce at 10 degrees celsius is heated in a heat exchanger by adding 121300 kj. calculate the outlet temperature of the applesauce. (hint: heat capacity for applesauce is given at 32.8 degrees celsius. assume that this is constant and use this as the average.)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, murtaghliam1
Word equation for k(s) +h2o(l) yield koh (aq) + h2
Answers: 3


Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
Do you know the correct answer?
Amixture of 454 kg of applesauce at 10 degrees celsius is heated in a heat exchanger by adding 12130...
Questions in other subjects:

Mathematics, 29.08.2019 16:40

Mathematics, 29.08.2019 16:40

Biology, 29.08.2019 16:40




Mathematics, 29.08.2019 16:40


Mathematics, 29.08.2019 16:40

Physics, 29.08.2019 16:40


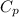 ) of apple sauce at
) of apple sauce at 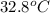 = 4.0177
= 4.0177 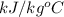
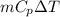
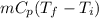
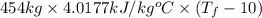
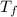 =
=