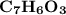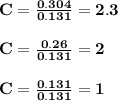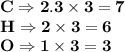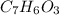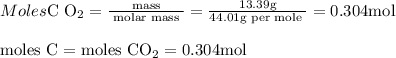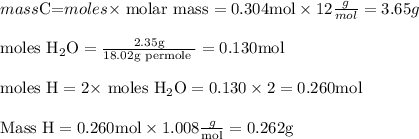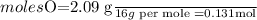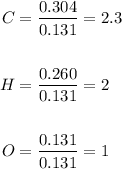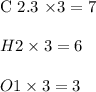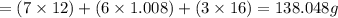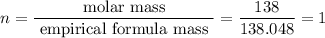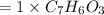Chemistry, 24.09.2019 05:10, Justadumbemo
6.0 g of a certain compound x, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 138 g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured:
carbon dioxide - 13.39 g
water - 2.35 g
use this information to find the molecular formula of x.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
Do you know the correct answer?
6.0 g of a certain compound x, known to be made of carbon, hydrogen and perhaps oxygen, and to have...
Questions in other subjects:

History, 19.11.2020 05:10


English, 19.11.2020 05:10

Mathematics, 19.11.2020 05:10

Geography, 19.11.2020 05:10


Mathematics, 19.11.2020 05:10


Mathematics, 19.11.2020 05:10

Mathematics, 19.11.2020 05:10