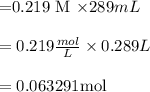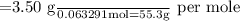Chemistry, 24.09.2019 04:20, dezmondpowell
3.50 g of an unknown base is dissolved in 289 ml of water and has a concentration of 0.219 m. what is the the identification of the unknown base.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1


Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 14:20, kekecantonxox121
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1
Do you know the correct answer?
3.50 g of an unknown base is dissolved in 289 ml of water and has a concentration of 0.219 m. what i...
Questions in other subjects:



Mathematics, 01.12.2020 17:00





Mathematics, 01.12.2020 17:00

Mathematics, 01.12.2020 17:00


 Volume
Volume