
Chemistry, 24.09.2019 03:20, haydencheramie
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen molecules) into the engine. consider a car that is using 100% ethanol, c2h5oh, as fuel. if your engine intakes 4.73 l of air per minute at 1.00 atm and 25ºc, what is the maximum volume of ethanol (0.789 g/ml) that can be burned per minute? hint: you can ignore the "per minute" information because both the ethanol and air are being quantified per minute. enter your answer to three significant figures in units of ml.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 22.06.2019 22:30, needhelpasap8957
Why is the bottom layer of a trophic pyrimid the
Answers: 2
Do you know the correct answer?
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen...
Questions in other subjects:



Social Studies, 18.12.2020 21:10

Mathematics, 18.12.2020 21:10

Business, 18.12.2020 21:10

Mathematics, 18.12.2020 21:10

Mathematics, 18.12.2020 21:10



History, 18.12.2020 21:10


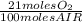 = 0,0406 moles O₂
= 0,0406 moles O₂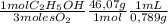 = 0,895 mL of ethanol per minute
= 0,895 mL of ethanol per minute




