
Chemistry, 24.09.2019 01:20, RickandMorty420710
Agas of potassium chlorate molecules kclo3 all decompose into potassium chloride, kcl, and diatomic oxygen, o2. the products and reactants are in a closed container and can all be treated as ideal gases. a. fill in the smallest possible integers that allows the stoichiometry of the reaction equation to be correct: __ kclo3 → kcl o2b. if there are n molecules of potassium chlorate in the initial state, how many product molecules are there

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 23.06.2019 00:30, terryg4397
Fred is studying a substance that is made out of only one element. this means that
Answers: 1

Chemistry, 23.06.2019 01:30, joyelewis58
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
Do you know the correct answer?
Agas of potassium chlorate molecules kclo3 all decompose into potassium chloride, kcl, and diatomic...
Questions in other subjects:

Advanced Placement (AP), 31.08.2021 20:10




Mathematics, 31.08.2021 20:10


Arts, 31.08.2021 20:10




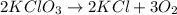
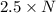 molecules of product.
molecules of product.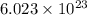 of particles.
of particles.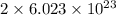 molecules of reactant give
molecules of reactant give 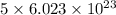 molecules of product
molecules of product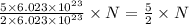 molecules of product.
molecules of product.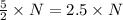 molecules of product are there.
molecules of product are there.



