Chemistry, 23.09.2019 21:10, sparky1234
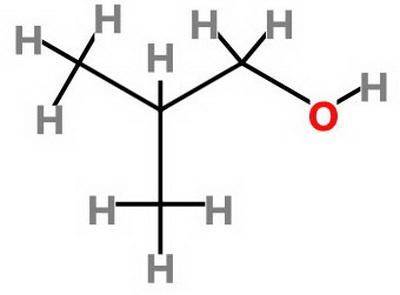
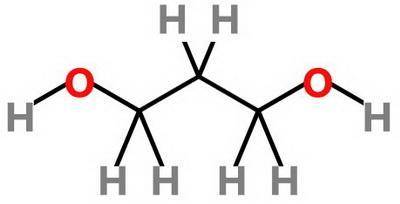
Which statement about 2‑methyl‑1‑propanol, (ch3)2chch2oh , and 1,3‑propanediol, hoch2ch2ch2oh is true? 2‑methyl‑1‑propanol is more soluble in water than 1,3‑propanediol because 2‑methyl‑1‑propanol has a smaller molecular mass. 2‑methyl‑1‑propanol is more soluble in water than 1,3‑propanediol because 2‑methyl‑1‑propanol forms fewer hydrogen bonds with water. 1,3‑propanediol is more soluble in water than 2‑methyl‑1‑propanol because 1,3‑propanediol has a smaller molecular mass. 1,3‑propanediol is more soluble in water than 2‑methyl‑1‑propanol because 1,3‑propanediol can form multiple hydrogen bonds with water.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1



Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
Do you know the correct answer?
Which statement about 2‑methyl‑1‑propanol, (ch3)2chch2oh , and 1,3‑propanediol, hoch2ch2ch2oh is tru...
Questions in other subjects:







Spanish, 13.04.2021 06:10