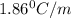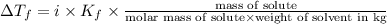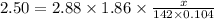Chemistry, 23.09.2019 18:10, pakabigail7116
What mass of na2so4 must be dissolved in 104 grams of water to lower the freezing point by 2.50 °c? the freezing point depression constant, kfp, of water is –1.86 °c/m. assume the van't hoff factor for na2so4 is 2.88.

Answers: 1
Similar questions

Chemistry, 14.07.2019 21:00, kingofguns1560
Answers: 1

Chemistry, 04.08.2019 07:00, achewitt3965
Answers: 1

Chemistry, 09.10.2019 15:30, mbarber3994
Answers: 3
Do you know the correct answer?
What mass of na2so4 must be dissolved in 104 grams of water to lower the freezing point by 2.50 °c?...
Questions in other subjects:

Spanish, 28.07.2019 09:30


Mathematics, 28.07.2019 09:30



Mathematics, 28.07.2019 09:30





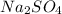 that must be dissolved is 6.89 grams.
that must be dissolved is 6.89 grams.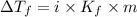
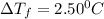 = Depression in freezing point
= Depression in freezing point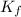 = freezing point constant =
= freezing point constant =