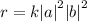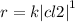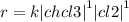Write the rate law
a)the reaction of oxygen with no2 is first order in oxygen and second order...

Write the rate law
a)the reaction of oxygen with no2 is first order in oxygen and second order in no2.
b)the reaction between no and cl2 is zero order in no and first order in cl2.
c)the reaction between cl2 and chloroform (chcl3) is first order in chcl3 and first order in cl2.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, irene4523
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake? o a. warm water has a greater heat of vaporization. ob. warm water evaporates more quickly. cool water evaporates more quickly. od. cool water has a greater heat of vaporization.
Answers: 1

Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 04:30, only1cache
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3
Do you know the correct answer?
Questions in other subjects:






Mathematics, 25.02.2020 01:45


Computers and Technology, 25.02.2020 01:45

History, 25.02.2020 01:45

Biology, 25.02.2020 01:45