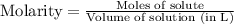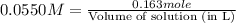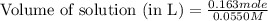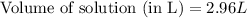Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, ddmoorehouseov75lc
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 23.06.2019 00:00, tonimgreen17p6vqjq
The graph indicates the running route for tobias. which best describes his run? from time 0 to 6, he went fast and then slowed down. from time 6 to 10, he was at his slowest. from time 12 to 14, he went very slow. from time 14 to 18, he went toward the starting point.
Answers: 2

Chemistry, 23.06.2019 02:30, ggpro4life3000
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2

Chemistry, 23.06.2019 07:00, MathChic68
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
Do you know the correct answer?
How many liters of a 0.0550 m naf solution contain 0.163 moles of naf?...
Questions in other subjects:

Mathematics, 10.07.2019 00:30

Mathematics, 10.07.2019 00:30



Mathematics, 10.07.2019 00:30

Chemistry, 10.07.2019 00:30




Social Studies, 10.07.2019 00:30