
Chemistry, 22.09.2019 03:20, shealynh52
Considering the limiting reactant, what is the mass of iron produced from 80.0 g of iron(ii)oxide (71.55 g/mol) and 20.0 g of magnesium metal? feof)+ mg() fe)mgo6) a) 62

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1



Do you know the correct answer?
Considering the limiting reactant, what is the mass of iron produced from 80.0 g of iron(ii)oxide (7...
Questions in other subjects:

Physics, 19.11.2020 23:30





Arts, 19.11.2020 23:30

Computers and Technology, 19.11.2020 23:30

English, 19.11.2020 23:30


English, 19.11.2020 23:30


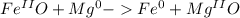
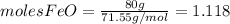 and
and  the molecular weight of Mg (24.305) can be readed in the periodic table of elements.so we divide the moles by stoichiometry number (number in front of each compound in the equation) in this case is 1 for both reactants (that is we need 1 mol of FeO and 1 mol of Mg to produce 1 mol of Fe).The lower number obtained was 0.823 for Mg, so Mg is the limiting reactant.
the molecular weight of Mg (24.305) can be readed in the periodic table of elements.so we divide the moles by stoichiometry number (number in front of each compound in the equation) in this case is 1 for both reactants (that is we need 1 mol of FeO and 1 mol of Mg to produce 1 mol of Fe).The lower number obtained was 0.823 for Mg, so Mg is the limiting reactant. ). To convert from mol of Fe to grams of Fe we would multiply by the molecular weight of Fe
). To convert from mol of Fe to grams of Fe we would multiply by the molecular weight of Fe 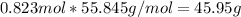 (molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron
(molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron



