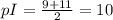Chemistry, 21.09.2019 23:10, mendezmarco2004
Suppose that you have an amino acid with three dissociated groups (a-carboxyl group, a-amino group and basic r-group), with the pkas 3, 9, and 11.
b. what is the pi
c. what is the net charge at ph 6

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 05:30, sethjohnson386pbnm3x
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3


Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
Do you know the correct answer?
Suppose that you have an amino acid with three dissociated groups (a-carboxyl group, a-amino group a...
Questions in other subjects:

Medicine, 13.04.2021 21:40

Mathematics, 13.04.2021 21:40


Mathematics, 13.04.2021 21:40

Mathematics, 13.04.2021 21:40


Chemistry, 13.04.2021 21:40

Mathematics, 13.04.2021 21:40

Mathematics, 13.04.2021 21:40

Chemistry, 13.04.2021 21:40