
Chemistry, 21.09.2019 23:10, ayoismeisalex
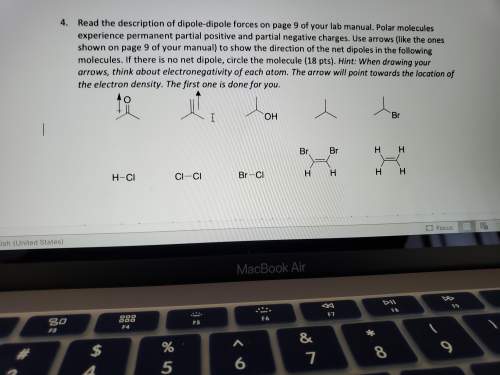
Read the description of dipole-dipole forces on page 9 of your lab manual. polar molecules experience permanent partial positive and partial negative charges. use arrows (like the ones shown on page 9 of your manual) to show the direction of the net dipoles in the following molecules. if there is no net dipole, circle the molecule (18 pts). hint: when drawing your arrows, think about electronegativity of each atom. the arrow will point towards the location of the electron density. the first one is done for you.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, lizbeth232001
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 21.06.2019 22:30, connienash95
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 08:00, danielhall
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1
Do you know the correct answer?
Read the description of dipole-dipole forces on page 9 of your lab manual. polar molecules experienc...
Questions in other subjects:





Biology, 06.09.2021 23:40

Mathematics, 06.09.2021 23:40


Mathematics, 06.09.2021 23:40

Mathematics, 06.09.2021 23:50

Mathematics, 06.09.2021 23:50






