
Chemistry, 21.09.2019 22:20, myleefaustin
Calculate the concentration of oh in a solution that contains 3910-4 m h30 at 25°c. identify the solution as acidic, basic or neutral oa) 2.6 10-11 m, acidic ob)26 10-11 m. basic o c) 3.9 x 10-4 m, neutral od) 2.7 * 10-2 m

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, notkeandre9
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Do you know the correct answer?
Calculate the concentration of oh in a solution that contains 3910-4 m h30 at 25°c. identify the sol...
Questions in other subjects:

History, 15.12.2021 06:00


Chemistry, 15.12.2021 06:00




Mathematics, 15.12.2021 06:00


Advanced Placement (AP), 15.12.2021 06:00


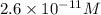 , acidic
, acidic![pH=-\log [H^+]](/tpl/images/0250/4594/37e81.png)
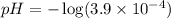
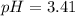
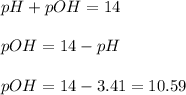
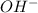 concentration.
concentration.![pOH=-\log [OH^-]](/tpl/images/0250/4594/1fac1.png)
![10.59=-\log [OH^-]](/tpl/images/0250/4594/21b6f.png)
![[OH^-]=2.6\times 10^{-11}M](/tpl/images/0250/4594/83ce7.png)




