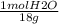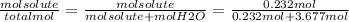Chemistry, 21.09.2019 21:30, floodlife4223
An aqueous solution is listed as being 33.8% solute by mass with a density of 1.15 g/ml, the molar mass of the solute is 145.6 g/mol and the molar mass of water is 18.0 g/mol. a) what is the molality of the solution? b) what is the mole fraction of the solute?

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Do you know the correct answer?
An aqueous solution is listed as being 33.8% solute by mass with a density of 1.15 g/ml, the molar m...
Questions in other subjects:

Biology, 15.12.2020 01:00

Biology, 15.12.2020 01:00

English, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00




Computers and Technology, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

English, 15.12.2020 01:00


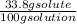 x
x 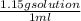 x
x 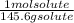 x
x 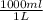
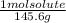 = 0.232 mol
= 0.232 mol