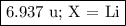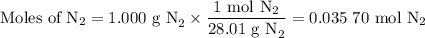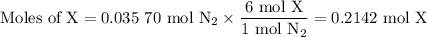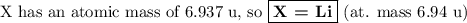Chemistry, 21.09.2019 07:30, MadiAbbott798
Nitrogen reacts with a metal to form a compound in which there are three atoms of the metal for each atom of nitrogen. if 1.486
g of the metal reacts with 1.000 g of nitrogen, what is the calculated atomic mass of the metal?
use your calculated atomic mass to identify the metal. (for your answer, input the proper chemical symbol for element x.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3


Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
Do you know the correct answer?
Nitrogen reacts with a metal to form a compound in which there are three atoms of the metal for each...
Questions in other subjects:

Mathematics, 25.06.2019 11:00


Mathematics, 25.06.2019 11:00


Physics, 25.06.2019 11:00

History, 25.06.2019 11:00



Biology, 25.06.2019 11:00

Mathematics, 25.06.2019 11:00