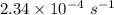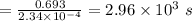Chemistry, 21.09.2019 05:30, lovely8458
The isomerization of methylisonitrile to acetonitrilech3nc(g)→ch3cn(g)is first order in ch3nc . the rate constant for the reaction is 2.34×10−4 s−1 at 489 k .the half-life of the reaction when the initial {\rm [ch_3nc]} is 0.030 m is {\rm s}.a. 1.43×105b. 3.37×10−4c. 4.28×103d. 2.14×103e. 2.96×103

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, rndschopplein
Achemical reaction (also known as a chemical change) produces substances that are chemically different from the starting materials. an example of a chemical reaction is the formation of water from hydrogen and oxygen gas. in a physical change, a substance changes its physical appearance but not its chemical identity. an example of physical change is the formation of liquid water from solid water, a familiar process called melting. physically, liquid water looks very different from solid water (ice) but the chemical identity, water, is the same for both. which of following changes that affect the composition of our atmosphere involve physical changes and which involve chemical reactions? oxygen gas changes to ozone during thunderstorms carbon dioxide is produced by the combustion of gasoline in an automobile engine. when coal, oil, and natural gas are decomposed in landsills they produce methane gas. freezing rain develops when a warm air mass overrides a cold air mass. fog forms from water vapor when the temperature drops below the dew point
Answers: 1

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1


Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
Do you know the correct answer?
The isomerization of methylisonitrile to acetonitrilech3nc(g)→ch3cn(g)is first order in ch3nc . the...
Questions in other subjects:

Mathematics, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

English, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01

English, 17.09.2020 01:01

English, 17.09.2020 01:01

Mathematics, 17.09.2020 01:01


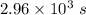
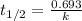
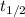 = Half life
= Half life