
The elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 690 k is 1.27 × 101 m-1s-1. the reaction half-life at this temperature when [no2]0 = 0.45 m is s. the elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 690 k is 1.27 × 101 m-1s-1. the reaction half-life at this temperature when [no2]0 = 0.45 m is s. 0.17 18 5.7 0.054 0.079

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, cordovamaria22
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 17:40, Snowball080717
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
Do you know the correct answer?
The elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 6...
Questions in other subjects:

Chemistry, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01






Mathematics, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01


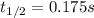
![\frac{1}{[NO_2]}=kt+ \frac{1}{[NO_2]_0}](/tpl/images/0248/0810/446e1.png)
![[NO_2]=\frac{[NO_2]_0}{2} \\](/tpl/images/0248/0810/70bce.png)
![t_{1/2}=\frac{1}{k[NO_2]_0} \\t_{1/2}=\frac{1}{(1.27x10^1\frac{L}{mol*s} )(0.45 \frac{mol}{L} )} \\\\t_{1/2}=0.175s](/tpl/images/0248/0810/f2ea3.png)




