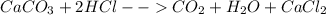Chemistry, 20.09.2019 23:30, sanchezp0821
Tums is a popular remedy for acid indigestion. a typical tums tablet contains calcium carbonate plus some inert substances. when ingested, it reacts with the gastric juice (hydrochloric acid) in the stomach to give off carbon dioxide gas. when a 1.367−g tablet reacted with 40.00 ml of hydrochloric acid (density = 1.140 g/ml), carbon dioxide gas was given off, and the resulting solution weighed 46.699 g. calculate the number of liters of carbon dioxide gas released if its density is 1.81 g/l.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, DarcieMATHlin2589
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1



Do you know the correct answer?
Tums is a popular remedy for acid indigestion. a typical tums tablet contains calcium carbonate plus...
Questions in other subjects:


Social Studies, 08.12.2021 07:10



English, 08.12.2021 07:10





Biology, 08.12.2021 07:10