
Chemistry, 20.09.2019 17:30, student0724
Akcl solution is prepared by dissolving 40.0 g kcl (molar mass = 74.55 g/mol) in 250.0 g of water (molar mass = 18.01 g/mol) at 25°c. what is the vapor pressure of the solution if the vapor pressure of water at 25°c is 23.76 mm hg?
a) 20.5 mm hg
b) 22.1 mm hg
c) 22.9 mm hg
d) 24.7 mm hg

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 02:50, giiffnlojd
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 08:00, celestemaria0727
What are the similarities of physical and chemical change ?
Answers: 1

Do you know the correct answer?
Akcl solution is prepared by dissolving 40.0 g kcl (molar mass = 74.55 g/mol) in 250.0 g of water (m...
Questions in other subjects:

Mathematics, 23.02.2021 19:20

Biology, 23.02.2021 19:20


English, 23.02.2021 19:20




Mathematics, 23.02.2021 19:20

English, 23.02.2021 19:20

Mathematics, 23.02.2021 19:20


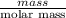
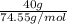
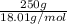
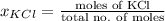
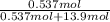
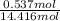
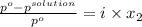
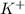 and
and 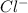 .
.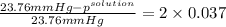
 = 22.1 mm Hg
= 22.1 mm Hg



