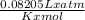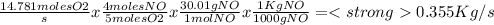Chemistry, 20.09.2019 17:10, DrizzyN2899
Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. the first step in its synthesis is the oxidation of ammonia. in this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water.
suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 645. liters per second of dioxygen are consumed when the reaction is run at 195.oc and 0.88 atm. calculate the rate at which nitrogen monoxide is being produced. give your answer in kilograms per second. be sure your answer has the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1


Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1
Do you know the correct answer?
Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. the first...
Questions in other subjects:

Mathematics, 16.09.2019 23:20



Mathematics, 16.09.2019 23:20


Mathematics, 16.09.2019 23:20

History, 16.09.2019 23:20

Social Studies, 16.09.2019 23:20