
Chemistry, 20.09.2019 04:10, brownw2005
the table compares the number of electrons in two unknown neutral atoms.
comparison of electrons
atom number of electrons
a 9
d 11
use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? (5 points)
both are unreactive.
both are highly reactive.
a is unreactive and d is reactive.
a is reactive and d is unreactive.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
Do you know the correct answer?
the table compares the number of electrons in two unknown neutral atoms.
comparison of electro...
comparison of electro...
Questions in other subjects:




Mathematics, 03.08.2019 18:30

Mathematics, 03.08.2019 18:30


Health, 03.08.2019 18:30

Mathematics, 03.08.2019 18:30


Social Studies, 03.08.2019 18:30


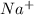 (2, 8)
(2, 8)
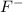 (2, 8)
(2, 8)




