
Valproic acid, used to treat seizures and bipolar disorder, is composed of c, h, and o. a 0.165-g sample is combusted to produce 0.166 g of water and 0.403 g of carbon dioxide. what is the empirical formula for valproic acid? if the molar mass is 144 g> mol, what is the molecular formula?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1


Do you know the correct answer?
Valproic acid, used to treat seizures and bipolar disorder, is composed of c, h, and o. a 0.165-g sa...
Questions in other subjects:



History, 21.12.2020 20:20




English, 21.12.2020 20:20



History, 21.12.2020 20:20


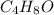
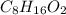
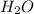 = 0.166 g /18 g/mol = 0.00922 moles
= 0.166 g /18 g/mol = 0.00922 moles
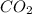 = 0.403 g /44.01 g/mol = 0.009157 moles
= 0.403 g /44.01 g/mol = 0.009157 moles




