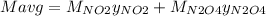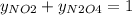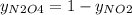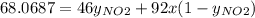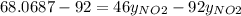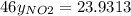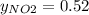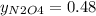Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, blakeboy3148
Draw the more stable lewis dot structure for bro2-
Answers: 2


Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3
Do you know the correct answer?
Nitrogen dioxide (no2) cannot be obtained in a pure form in the gas phase because it exists as a mix...
Questions in other subjects:


History, 26.05.2020 21:57




Mathematics, 26.05.2020 21:57

Health, 26.05.2020 21:57

English, 26.05.2020 21:57


Mathematics, 26.05.2020 21:57


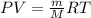
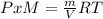
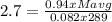
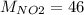 g/mol and
g/mol and 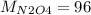 g/mol. Calling y the molar fraction:
g/mol. Calling y the molar fraction: