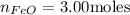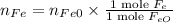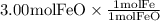Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, kawaiiblurainbow
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 02:10, sativataurus
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2
Do you know the correct answer?
The reaction between iron(ii) oxide and carbon monoxide produces iron and carbon dioxide. how many m...
Questions in other subjects:


Health, 03.09.2020 09:01

Mathematics, 03.09.2020 09:01

English, 03.09.2020 09:01

Geography, 03.09.2020 09:01



English, 03.09.2020 14:01


Mathematics, 03.09.2020 14:01