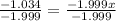Chemistry, 19.09.2019 16:30, trinityhayes347
The element silver consists of two isotopes 107ag (atomic mass = 106.905) and 109ag (atomic mass = 108.904). the average atomic mass of silver is 107.87. the natural abundances of 107ag and 109ag are % and %, respectively.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 04:40, yayamcneal05
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
Do you know the correct answer?
The element silver consists of two isotopes 107ag (atomic mass = 106.905) and 109ag (atomic mass = 1...
Questions in other subjects:




History, 15.01.2020 23:31

Biology, 15.01.2020 23:31