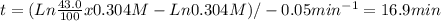Chemistry, 19.09.2019 16:30, buddyshaw76
The decomposition of a to b is a first-order reaction with a half-life of 14.2 min: a → 2b if the initial concentration of a is 0.304 m, how long will it take for the concentration of a to decrease by 43.0 %?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 19:00, cindyroxana229
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 23.06.2019 02:40, sherlock19
How can a mixture of salt water be separated into salt and water
Answers: 1
Do you know the correct answer?
The decomposition of a to b is a first-order reaction with a half-life of 14.2 min: a → 2b if the i...
Questions in other subjects:


Mathematics, 04.12.2020 22:50



Mathematics, 04.12.2020 22:50





History, 04.12.2020 22:50


![Ln [A] = -k.t + Ln [A]_{0}](/tpl/images/0243/4301/30b90.png)
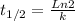
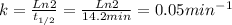
![[A] = \frac{43.0}{100}x[A]_{0}](/tpl/images/0243/4301/7dcf6.png)
![Ln \frac{43.0}{100} [A]_{0} = -k.t + Ln [A]_{0}](/tpl/images/0243/4301/2b903.png)
![(Ln \frac{43.0}{100} [A]_{0} - Ln [A]_{0} ) / -k = t](/tpl/images/0243/4301/1d19e.png)