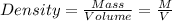Chemistry, 19.09.2019 07:30, issagershome
Two objects each have a mass of 5.0 g. one had a density of 2.7 g. and the other has a density of 8.4 g. which object has a larger volume?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 15:30, kfull6027
The amount of iron in ore can be quantitatively determined by titrating a solution of the unknown with a standard solution of dichromate, cr2o72−. the net ionic equation is 6fe2+(aq)+cr2o72−(aq)+14h+(aq)→6fe3 +(aq)+2cr3+(aq)+7h2o(aq) part a the titration of 25.0 ml of an iron(ii) solution required 18.0 ml of a 0.230 m solution of dichromate to reach the equivalence point. what is the molarity of the iron(ii) solution?
Answers: 1

Chemistry, 23.06.2019 18:00, ashleyremon
What would make it possible for magnesium atom to have a noble gas configuration
Answers: 3
Do you know the correct answer?
Two objects each have a mass of 5.0 g. one had a density of 2.7 g. and the other has a density of 8....
Questions in other subjects:

Mathematics, 29.08.2019 07:50

History, 29.08.2019 07:50

English, 29.08.2019 07:50


English, 29.08.2019 07:50


Mathematics, 29.08.2019 07:50




 has a larger volume
has a larger volume