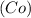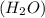Chemistry, 19.09.2019 03:30, cassandramanuel
while elements and compounds can both be classified as pure substances because they have distinct properties and composition, cannot be decomposed further.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 18:30, robjaykay
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 23.06.2019 00:20, cmflores3245
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
Do you know the correct answer?
while elements and compounds can both be classified as pure substances because they have distinct pr...
Questions in other subjects:

Mathematics, 16.12.2020 19:00

Mathematics, 16.12.2020 19:00


English, 16.12.2020 19:00

Mathematics, 16.12.2020 19:00

Biology, 16.12.2020 19:00

Physics, 16.12.2020 19:00

Chemistry, 16.12.2020 19:00

Mathematics, 16.12.2020 19:00