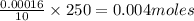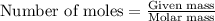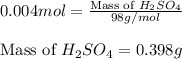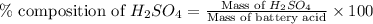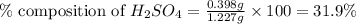Asample of battery acid is to be analyzed for its sulfuric acid content. a 1.00-ml sample weighs 1.227 g . this 1.00-ml sample is diluted to 250.0 ml, and 10.00 ml of this diluted acid requires 35.05 ml of 4.462×10−3 m ba(oh)2 for its titration. what is the mass percent of h2so4 in the battery acid?

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
Do you know the correct answer?
Asample of battery acid is to be analyzed for its sulfuric acid content. a 1.00-ml sample weighs 1.2...
Questions in other subjects:


Mathematics, 20.08.2019 18:30






English, 20.08.2019 18:30

Geography, 20.08.2019 18:30


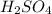 in the battery acid is 31.9 %
in the battery acid is 31.9 %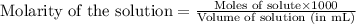
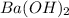 solution =
solution = 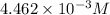
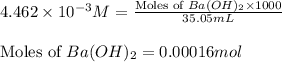
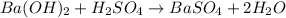
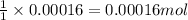 of sulfuric acid.
of sulfuric acid.