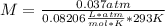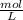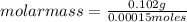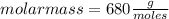Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, shonnybenskin8
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
Do you know the correct answer?
Asolution containing 0.102 g of an unknown compound dissolved in 100. ml of water has an osmotic pre...
Questions in other subjects:

Mathematics, 09.09.2020 14:01

Mathematics, 09.09.2020 14:01

Mathematics, 09.09.2020 14:01

Mathematics, 09.09.2020 14:01

History, 09.09.2020 14:01

Mathematics, 09.09.2020 14:01

Mathematics, 09.09.2020 14:01

Mathematics, 09.09.2020 14:01

Mathematics, 09.09.2020 14:01

English, 09.09.2020 14:01


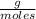
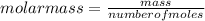
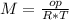
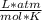 T=20 °C= 293°K (0°C=273 °K)
T=20 °C= 293°K (0°C=273 °K)