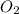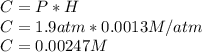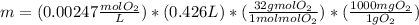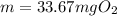Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
Do you know the correct answer?
What mass of oxygen gas, in mg, is dissolved in 4.26×102 ml of water when the partial pressure of ox...
Questions in other subjects:

Mathematics, 03.03.2021 03:50


English, 03.03.2021 03:50

Spanish, 03.03.2021 03:50



Mathematics, 03.03.2021 03:50

Biology, 03.03.2021 03:50