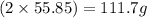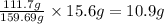Chemistry, 18.09.2019 17:10, michellemonroe012305
A29.7 g sample of iron ore is treated as follows. the iron in the sample is all converted by a series of chemical reactions to fe₂o₃. the mass of fe₂o₃ is measured to be 15.6 g. what was the mass of iron in the sample of ore? answer in units of g.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, isalih7256
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1


Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 00:30, emilylizbeth12334
Which of the following best describes technology a. something created for only scientists to use b. the method of thinking that scientists use. c. the application of engineering to create useful products. c. a scientific idea
Answers: 1
Do you know the correct answer?
A29.7 g sample of iron ore is treated as follows. the iron in the sample is all converted by a serie...
Questions in other subjects:










Physics, 02.08.2019 06:30