
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o2) are carbon dioxide (co2) and water (h2o).
a mass of 16.74 g for an unknown fuel was combusted in a reaction vessel containing an unknown amount of oxygen. at the end of the reaction, there still remained 16.70 g of the fuel as well as 0.0654 g of water and 0.1198 g of carbon dioxide. the oxygen was completely consumed during the reaction.
how many molecules of oxygen gas were initially present in the reaction vessel?
me, i will be very . the topic is limiting reagents and theoretical yields.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 21:40, k3rbycalilung
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 23.06.2019 00:00, PineappleDevil889
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 07:00, jboii11
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
Do you know the correct answer?
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o...
Questions in other subjects:

English, 28.09.2020 14:01

Geography, 28.09.2020 14:01

Mathematics, 28.09.2020 14:01


Physics, 28.09.2020 14:01



History, 28.09.2020 14:01



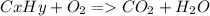
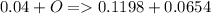
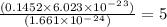 molecules
molecules




