
Chemistry, 18.09.2019 05:20, stephaniero6
Asample of liquefied natural, lng, from alaska has the following molar composition: 3.9% c2h6, 1.1% c3h8, 1.1% co2, and the rest ch4. determine (a) the average molecular weight of the lng mixture. (b) the weight fraction of ch4 in the mixture. (c) the lng is heated to 422 k and 148 kpa, and vaporizes completely. estimate the density of the gas mixture under these conditions.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 15:00, Zagorodniypolina5
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Do you know the correct answer?
Asample of liquefied natural, lng, from alaska has the following molar composition: 3.9% c2h6, 1.1%...
Questions in other subjects:


Biology, 19.04.2020 06:40

Geography, 19.04.2020 06:40


Mathematics, 19.04.2020 06:41


Mathematics, 19.04.2020 06:41


Mathematics, 19.04.2020 06:42




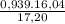 ×100 = 87,57%
×100 = 87,57%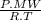 = δ
= δ





