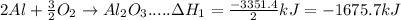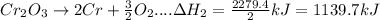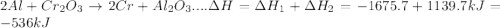In the following overall chemical reaction, aluminum displaces chromium from chromium(iii) oxide and forms aluminum oxide.
2 al(s) + cr2o3(s) → 2 cr(s) + al2o3(s); δh = ?
find the change in enthalpy for this reaction, using hess' law and the enthalpy changes for the reactions given below.
(1a) 4 al(s) + 3 o2(g) → 2 al2o3(s); δh = −3351.4 kj
(2a) 4 cr(s) + 3 o2(g) → 2 cr2o3(s); δh = −2279.4 kj

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, Tyrant4life
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2

Chemistry, 23.06.2019 06:20, Lindsay882
Type the correct answer in each box. balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1


Chemistry, 23.06.2019 08:50, leah5981
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas. caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible. relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3
Do you know the correct answer?
In the following overall chemical reaction, aluminum displaces chromium from chromium(iii) oxide and...
Questions in other subjects:

History, 21.11.2020 14:00

Mathematics, 21.11.2020 14:00

Social Studies, 21.11.2020 14:00

Mathematics, 21.11.2020 14:00


Social Studies, 21.11.2020 14:00





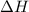 is an additive property. hence value of
is an additive property. hence value of